It’s been quite a roller coaster couple of weeks, Covid-wise, with good news and bad news making headlines
daily.
The bad news locally, of course, is that Humboldt leapfrogged from yellow to red (skipping orange), and then—last Tuesday, after a weekend that brought 56 new cases—landed in the purple (“widespread”) tier. Purple is no longer the color once reserved for royalty (because of its scarcity—it took 250,000 tiny sea snails, Bolinus brandaris, to make one ounce of purple dye); now it’s the color of the strictest state regulations designed to prevent the spread of Covid. Humboldt is in good—or bad, I guess—company, sharing its new designation with 41 of the state’s 58 counties. Some 37 million of us are now in purple, meaning that you won’t be able to eat or drink inside, and gyms, movie theaters, nonessential business offices and houses of worship are closed. Stores can open at 25% capacity, grocery stores at 50%.
The good news is that no less than three candidate vaccines appear to be on track for early approval, with the first shots to be given—probably to healthcare workers—before the end of the year. Which came as a total surprise to many of us who have been following the saga of vaccine development, and who didn’t expect such early and effective results. “Effective” here means “efficacy,” that is, the statistical probability that a vaccinated person won’t get Covid if they’re exposed to it. The FDA’s bar to green-light a vaccine, to “prevent disease or decrease its severity in at least 50 percent of people who are vaccinated,” now seems ridiculously low, with all three vaccines coming in around 90% in trials involving tens of thousands of people.
The earliest announcement came from Pfizer Inc. and its German partner BioNTech, which claimed a 90% efficacy from a study in which 38,000 people were either given the vaccine or a saline placebo. Pfizer says that, assuming emergency approval by the FDA, they’ll be able to ship 25 million doses in December, ramping up to over 30 million in subsequent months. But—and it’s a huge but—their vaccine is vulnerable to storage temperatures warmer than minus 80 degrees Celsius (nearly as low as the coldest ever recorded, minus –89 C, in Antarctica in 2010). Pfizer says they’re on top of their deep-freeze problem, but this requirement is nearly unprecedented: standard vaccine storage calls for temperatures of around 9 C. (Actually, some African countries may be better off than we are, since hundreds of thousands of people in the Democratic Republic of Congo, South Sudan, Uganda, Rwanda and Burundi have been vaccinated against the Ebola virus; the Merck Ebola vaccine has to be stored at –70 C.) Cost: $20/dose, with two doses three weeks apart needed.
Second up was Moderna’s candidate, which, like Pfizer-BioNTech, uses a novel RNA approach (unlike traditional vaccines that rely on dead or weakened viruses to alert the immune system). Moderna claims to have a 94.5% efficacy rate. The cold storage requirements for their vaccine are far less stringent than Pfizer’s: it can last for up to six months at –20 C, and 30 days in a regular fridge. Cost: $32-37 per dose, two doses required.
And third, best of all, came last week’s announcement from the Oxford University-AstraZeneca collaboration, which claimed 90% efficacy for their more traditional vaccine, a genetically modified version of a virus that typically causes colds in chimpanzees. Oddly, that 90% was for participants who were first given a half-dose, then, a month later, a full dose of the vaccine. (Volunteers given two full doses only had a 60% efficacy.) The really good news comes in three parts: (1) the vaccine seems to be stable for six months at normal fridge temperatures; (2) AstraZeneca claims it can deliver three billion doses by the end of 2021 (exceeding Pfizer and Moderna’s combined output); (3) they say they’ll provide the vaccine at cost, around $3 per dose.
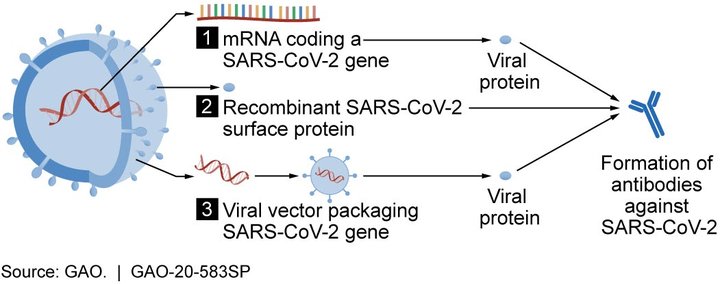
Pfizer-BioNTech and Moderna both use a novel mRNA strategy 1. Oxford-AstraZeneca uses the more traditional strategy 3, in this case a modified chimpanzee adenovirus vector.
Caveats: Results for all these candidate vaccines (and many others in development) are preliminary; none have yet been approved by the FDA; no long-term results are available (obviously), meaning that repeat shots may be needed at, say, yearly intervals; and very old or very young people weren’t included in the initial trials.
CLICK TO MANAGE